Are you curious about one of the most influential and fundamental principles in quantum physics? In Part 2 of our Quantum Physics series, we dive deep into the Heisenberg Uncertainty Principle, exploring its implications on the uncertainty of the properties of particles at the atomic level. Get ready to uncover how this concept has revolutionized our understanding of the universe.
In 1927, the German physicist Werner Heisenberg mathematically determined a formal inequality that stated the relation between the deterministic precision between the position of a particle and its momentum.
Heisenberg’s uncertainty principle states that “the more the precision with which the position of a particle can be determined, the lesser the precision with which its momentum can be determined and vice versa”, i.e. the error margins of these two properties with which it can be determined, remains conserved which is defined by the given equation.
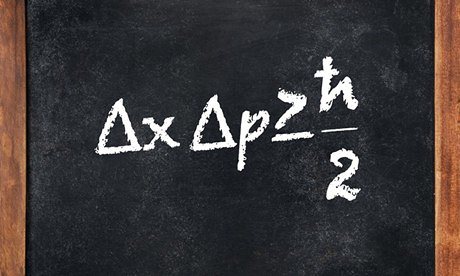
where Δx = uncertainty in position , and
Δp = uncertainty in momentum
This also tells us that if momentum is determined with utmost precision(Δp = 0), the position is indeterminable(Δx = ∞).
But what is the physical interpretation of such an expression?
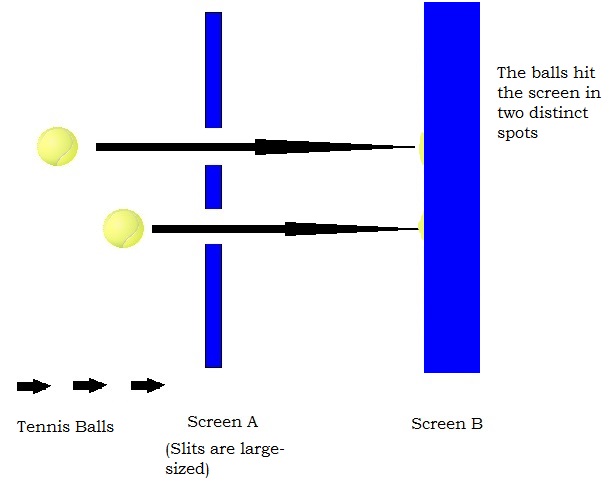
Let us refer to the previous experiment , where we used tennis balls against electrons to prove that electrons exhibits wave-like properties unlike macroscopic objects such as tennis balls as shown in Figure A. The truth is that both electrons and tennis balls exhibit dual properties of both matter and energy and lie within idealistic extremes of a typical wave(energy) and matter. The wave property of an object is inversely proportional to its particle property.
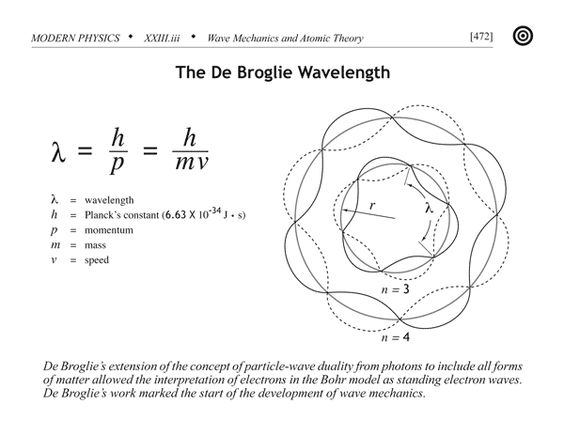
A tennis ball exhibits properties of a particle with much more prominence than it’s a wave properties. The more wavelength an object, the more is it’s wave-like nature. This wavelength known as De Broglie wavelength, in turn, depends on the momentum of the particle.
Note that a high momentum is associated with velocity and mass as
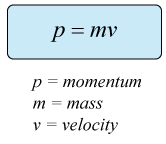
If the product of mass and velocity is overall high, then we can say that momentum is high. This results in a lower wave-like property. A tennis ball, is many times massive than an electron, and hence has a wavelength, of an order 10-34 , which is negligible. This is why, for all practical purpose, a tennis ball can be considered as matter, whose position can be perfectly determined.
A pure wave on the other hand, has no position and hence it’s momentum can be determined with certainty.
An electron has a wavelength of about 1 nm(10-3 m), which despite its apparently small size is a hundred billion times larger than the size of an atom which is responsible for its wave-like nature. That being said, an electron still has mass and the consequential uncertainty in position and momentum. Both, it wave and particle nature is observable in experiments.
To obtain a quantum particle(through the slits) that exhibit dual characteristics of matter and wave, we have to introduce both uncertainties in position and momentum.
Introducing uncertainty in position
Let us consider, a small area in an electron wave after passing through the slits. (If we were to consider the entire wave space as a whole i.e. the setup volume of Young’s experiment, we could predict with certainty, that the particle exists inside that chosen volume.Instead, we only select a small section of the volume where particle may or may not exist, thereby deliberately introducing uncertainty in position)
Introducing uncertainty in momentum
We mix multiple waves of different wavelength, and let them interfere with each other.
When waves interfere with each other, it loses its constant wavelength, along with the associated predictability of its momentum. This is because interfering waves, either amplify(through constructive interference), DE-amplify or nullify (through destructive constructive interference)the crests and troughs of a smooth wave as shown in Figure B.
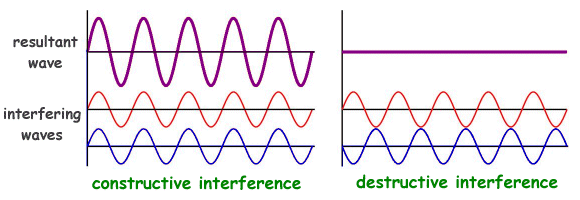
This change in wave structure is what introduces uncertainty in its momentum. The interfering wave pattern inside the fixed chosen volume(which is a sub-section of the expanse of the entire wave) has a certain uncertainty in both its position and momentum. Hence, it exhibits dual properties and can be termed as a quantum particle. Let us refer to the diagram discussed in one of the previous articles.
UNDERSTANDING HEISENBERG’S UNCERTAINTY PRINCIPLE IN CONTEXT OF YOUNG’S DOUBLE SLIT EXPERIMENT
When momentum is determinable
Before, hitting the first screen A, the particles were thrown in a general direction perpendicular to the screen’s surface with a fixed velocity. Now, because mass of an electron is known, its momentum is also therefore known. This introduces an infinite amount of uncertainty in its position.
When position is determinable
The reverse happens when the electron hits the screen B. Because of collision, its position is now known and hence, its momentum becomes indeterminable.
CONCLUSION AND REAL-LIFE APPLICATION
The world at the quantum level is not different from than the classical level. In the previous article, we have introduced quantum physics as an anomaly to the existing classical theories which are both intuitive and apparent. Although this simplified statement serves as an useful introductory point and helps us separate it out from the classical domain, it is important to understand accurately that at no scale, classical physics suddenly takes over the quantum principles or vice-versa, rendering the other useless in its own domain. This scale of being macroscopic or microscopic is purely subjective and depends on the observer. It is just that at relatively larger scale, it is easier to ignore the quantum principles and the error margin gets reduced significantly. To understand as to why Heisenberg’s principle is not apparent in real life, let us try to determine the position and momentum of a large object such as a vehicle. We can, of course, unlike an electron, determine both its position and momentum simultaneously, yet the precision with which we do so is nowhere near what is considered accurate at the quantum level. When we say, that a vehicle was traveling at 100 miles/hour , a mile away from a certain bus-stand, we are ignoring the errors in both these measurements, and thus are considering them to be accurate. If we were to get too precise, and decide to take measurement up to a hundred decimal places, we would be facing some problems. In attempt to measure a car’s position, we must stop in and sacrifice the accuracy of it’s velocity instead and obtain a result that is very precise. The value of velocity, if measured at all, might vary greatly from what it would actually be. Thus there is a trade-off between the error margins.
This is applicable to many more pairs of conjugates -where in order for something to be precisely measured, the accuracy of something else must be sacrificed instead.

3 thoughts on “Heisenberg’s Uncertainty Principle (Quantum Physics Part 2 )”